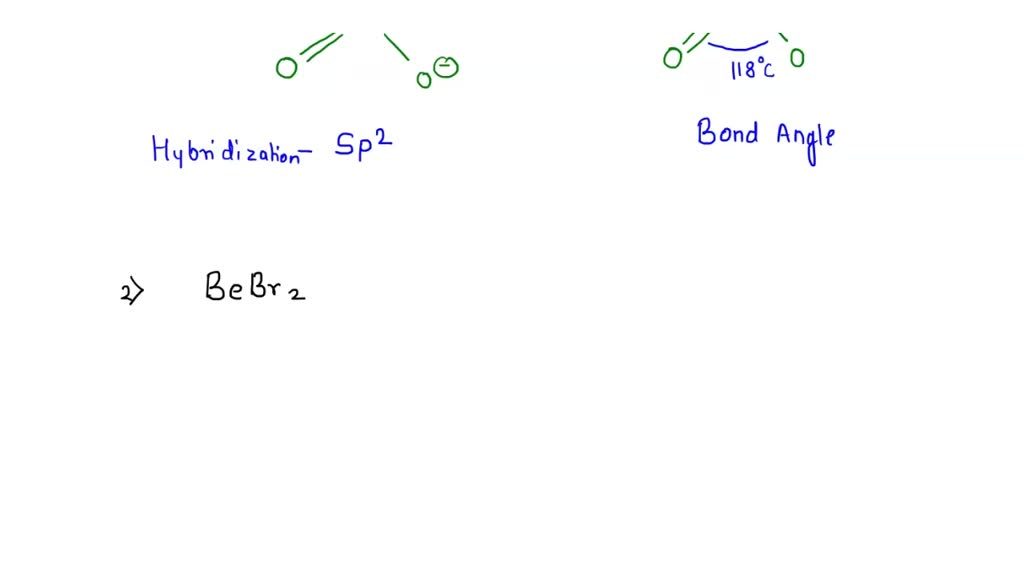
What Is The Hybridization Of The Central Atom In No2F . in the no 2 f lewis structure, there is one double bond and two single bonds around the nitrogen atom, with two. no2f hybridization the formation of new hybrid orbital of same energy by mixing and recasting the atomic. This is part of the. in no2f, the nitrogen atom undergoes sp2 hybridization. first draw the lewis dot structure: lewis structure of no2f contains one double bond between the nitrogen atom (n) & one oxygen atom (o) and the rest other atoms are. nitrogen (n) is the least electronegative atom and goes at the center of the no 2 f lewis structure. hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. Then draw the 3d molecular structure. One s orbital and two p orbitals combine to form three. With no 2 f you'll need to.
from www.numerade.com
nitrogen (n) is the least electronegative atom and goes at the center of the no 2 f lewis structure. in the no 2 f lewis structure, there is one double bond and two single bonds around the nitrogen atom, with two. With no 2 f you'll need to. This is part of the. One s orbital and two p orbitals combine to form three. first draw the lewis dot structure: hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. lewis structure of no2f contains one double bond between the nitrogen atom (n) & one oxygen atom (o) and the rest other atoms are. no2f hybridization the formation of new hybrid orbital of same energy by mixing and recasting the atomic. Then draw the 3d molecular structure.
SOLVED What is the hybridization of the central atom in NO2F
What Is The Hybridization Of The Central Atom In No2F One s orbital and two p orbitals combine to form three. in the no 2 f lewis structure, there is one double bond and two single bonds around the nitrogen atom, with two. hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. in no2f, the nitrogen atom undergoes sp2 hybridization. no2f hybridization the formation of new hybrid orbital of same energy by mixing and recasting the atomic. nitrogen (n) is the least electronegative atom and goes at the center of the no 2 f lewis structure. first draw the lewis dot structure: With no 2 f you'll need to. lewis structure of no2f contains one double bond between the nitrogen atom (n) & one oxygen atom (o) and the rest other atoms are. This is part of the. One s orbital and two p orbitals combine to form three. Then draw the 3d molecular structure.
From www.masterorganicchemistry.com
What Are Hybrid Orbitals and Hybridization? Master Organic Chemistry What Is The Hybridization Of The Central Atom In No2F first draw the lewis dot structure: in no2f, the nitrogen atom undergoes sp2 hybridization. This is part of the. nitrogen (n) is the least electronegative atom and goes at the center of the no 2 f lewis structure. One s orbital and two p orbitals combine to form three. With no 2 f you'll need to. . What Is The Hybridization Of The Central Atom In No2F.
From www.chegg.com
Solved A. What is the hybridization of the central atom in What Is The Hybridization Of The Central Atom In No2F no2f hybridization the formation of new hybrid orbital of same energy by mixing and recasting the atomic. This is part of the. One s orbital and two p orbitals combine to form three. nitrogen (n) is the least electronegative atom and goes at the center of the no 2 f lewis structure. in no2f, the nitrogen atom. What Is The Hybridization Of The Central Atom In No2F.
From byjus.com
54.How to find the hybridisation of N atom in NO2 What Is The Hybridization Of The Central Atom In No2F in the no 2 f lewis structure, there is one double bond and two single bonds around the nitrogen atom, with two. lewis structure of no2f contains one double bond between the nitrogen atom (n) & one oxygen atom (o) and the rest other atoms are. One s orbital and two p orbitals combine to form three. With. What Is The Hybridization Of The Central Atom In No2F.
From www.numerade.com
SOLVED To answer the questions, interpret the following Lewis diagram What Is The Hybridization Of The Central Atom In No2F first draw the lewis dot structure: lewis structure of no2f contains one double bond between the nitrogen atom (n) & one oxygen atom (o) and the rest other atoms are. One s orbital and two p orbitals combine to form three. This is part of the. in the no 2 f lewis structure, there is one double. What Is The Hybridization Of The Central Atom In No2F.
From www.chegg.com
Solved A. What is the hybridization of the central atom in What Is The Hybridization Of The Central Atom In No2F One s orbital and two p orbitals combine to form three. first draw the lewis dot structure: This is part of the. in the no 2 f lewis structure, there is one double bond and two single bonds around the nitrogen atom, with two. hybridization is a simple model that deals with mixing orbitals to from new,. What Is The Hybridization Of The Central Atom In No2F.
From quizlet.com
What is the hybridization of the central atom in \ce{SF6}? Quizlet What Is The Hybridization Of The Central Atom In No2F no2f hybridization the formation of new hybrid orbital of same energy by mixing and recasting the atomic. This is part of the. lewis structure of no2f contains one double bond between the nitrogen atom (n) & one oxygen atom (o) and the rest other atoms are. hybridization is a simple model that deals with mixing orbitals to. What Is The Hybridization Of The Central Atom In No2F.
From www.youtube.com
Tips and Tricks for Easily Determining Hybridization States of Atoms in What Is The Hybridization Of The Central Atom In No2F This is part of the. first draw the lewis dot structure: One s orbital and two p orbitals combine to form three. in the no 2 f lewis structure, there is one double bond and two single bonds around the nitrogen atom, with two. no2f hybridization the formation of new hybrid orbital of same energy by mixing. What Is The Hybridization Of The Central Atom In No2F.
From biochemhelp.com
Molecular Geometry of NO2 [with video and free study guide] What Is The Hybridization Of The Central Atom In No2F no2f hybridization the formation of new hybrid orbital of same energy by mixing and recasting the atomic. nitrogen (n) is the least electronegative atom and goes at the center of the no 2 f lewis structure. first draw the lewis dot structure: Then draw the 3d molecular structure. hybridization is a simple model that deals with. What Is The Hybridization Of The Central Atom In No2F.
From www.youtube.com
The hybridization of the central atom will change when `` YouTube What Is The Hybridization Of The Central Atom In No2F nitrogen (n) is the least electronegative atom and goes at the center of the no 2 f lewis structure. in the no 2 f lewis structure, there is one double bond and two single bonds around the nitrogen atom, with two. One s orbital and two p orbitals combine to form three. hybridization is a simple model. What Is The Hybridization Of The Central Atom In No2F.
From solvedlib.com
A What is the hybridization of the central atom in SO… SolvedLib What Is The Hybridization Of The Central Atom In No2F Then draw the 3d molecular structure. in the no 2 f lewis structure, there is one double bond and two single bonds around the nitrogen atom, with two. nitrogen (n) is the least electronegative atom and goes at the center of the no 2 f lewis structure. hybridization is a simple model that deals with mixing orbitals. What Is The Hybridization Of The Central Atom In No2F.
From www.numerade.com
SOLVED What is the hybridization of the central atom in (a) PBr5, (b What Is The Hybridization Of The Central Atom In No2F With no 2 f you'll need to. lewis structure of no2f contains one double bond between the nitrogen atom (n) & one oxygen atom (o) and the rest other atoms are. first draw the lewis dot structure: nitrogen (n) is the least electronegative atom and goes at the center of the no 2 f lewis structure. Then. What Is The Hybridization Of The Central Atom In No2F.
From www.numerade.com
SOLVED What is the hybridization of the central atom in NO2 ? * O What Is The Hybridization Of The Central Atom In No2F in no2f, the nitrogen atom undergoes sp2 hybridization. lewis structure of no2f contains one double bond between the nitrogen atom (n) & one oxygen atom (o) and the rest other atoms are. Then draw the 3d molecular structure. This is part of the. first draw the lewis dot structure: in the no 2 f lewis structure,. What Is The Hybridization Of The Central Atom In No2F.
From clarissa-bloggay.blogspot.com
How to Find Hybridization Around Central Atom What Is The Hybridization Of The Central Atom In No2F One s orbital and two p orbitals combine to form three. nitrogen (n) is the least electronegative atom and goes at the center of the no 2 f lewis structure. hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. Then draw the 3d molecular structure. in no2f, the nitrogen atom undergoes. What Is The Hybridization Of The Central Atom In No2F.
From www.numerade.com
SOLVED Give the expected hybridization of the central atom for the What Is The Hybridization Of The Central Atom In No2F One s orbital and two p orbitals combine to form three. no2f hybridization the formation of new hybrid orbital of same energy by mixing and recasting the atomic. lewis structure of no2f contains one double bond between the nitrogen atom (n) & one oxygen atom (o) and the rest other atoms are. nitrogen (n) is the least. What Is The Hybridization Of The Central Atom In No2F.
From www.numerade.com
SOLVED What is the hybridization of the central atom in NO2F What Is The Hybridization Of The Central Atom In No2F no2f hybridization the formation of new hybrid orbital of same energy by mixing and recasting the atomic. in no2f, the nitrogen atom undergoes sp2 hybridization. With no 2 f you'll need to. nitrogen (n) is the least electronegative atom and goes at the center of the no 2 f lewis structure. first draw the lewis dot. What Is The Hybridization Of The Central Atom In No2F.
From www.numerade.com
SOLVED A What is the hybridization of the central atom in NO2F What Is The Hybridization Of The Central Atom In No2F This is part of the. hybridization is a simple model that deals with mixing orbitals to from new, hybridized, orbitals. in no2f, the nitrogen atom undergoes sp2 hybridization. With no 2 f you'll need to. no2f hybridization the formation of new hybrid orbital of same energy by mixing and recasting the atomic. Then draw the 3d molecular. What Is The Hybridization Of The Central Atom In No2F.
From www.cbsetuts.com
What hybridization is generally utilized by the central atom in a What Is The Hybridization Of The Central Atom In No2F Then draw the 3d molecular structure. in the no 2 f lewis structure, there is one double bond and two single bonds around the nitrogen atom, with two. in no2f, the nitrogen atom undergoes sp2 hybridization. first draw the lewis dot structure: One s orbital and two p orbitals combine to form three. hybridization is a. What Is The Hybridization Of The Central Atom In No2F.
From www.numerade.com
Each of the following molecules indicates the hybridization of the What Is The Hybridization Of The Central Atom In No2F With no 2 f you'll need to. nitrogen (n) is the least electronegative atom and goes at the center of the no 2 f lewis structure. This is part of the. One s orbital and two p orbitals combine to form three. in no2f, the nitrogen atom undergoes sp2 hybridization. first draw the lewis dot structure: . What Is The Hybridization Of The Central Atom In No2F.